Protein-coding gene in the species Homo sapiens
| PPP1R12A |
|---|
 |
| Available structures |
|---|
| PDB | Ortholog search: PDBe RCSB |
|---|
| List of PDB id codes |
|---|
2KJY, 2MXR, 5HUZ |
|
|
| Identifiers |
|---|
| Aliases | PPP1R12A, M130, MBS, MYPT1, protein phosphatase 1 regulatory subunit 12A, GUBS |
|---|
| External IDs | OMIM: 602021; MGI: 1309528; HomoloGene: 1855; GeneCards: PPP1R12A; OMA:PPP1R12A - orthologs |
|---|
| Gene location (Human) |
|---|
 | | Chr. | Chromosome 12 (human)[1] |
|---|
| | Band | 12q21.2-q21.31 | Start | 79,773,563 bp[1] |
|---|
| End | 79,935,460 bp[1] |
|---|
|
| Gene location (Mouse) |
|---|
 | | Chr. | Chromosome 10 (mouse)[2] |
|---|
| | Band | 10 D1|10 56.33 cM | Start | 107,998,054 bp[2] |
|---|
| End | 108,120,336 bp[2] |
|---|
|
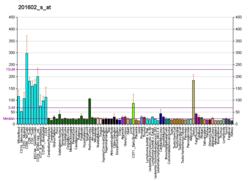
| RNA expression pattern |
|---|
| Bgee | | Human | Mouse (ortholog) |
|---|
| Top expressed in | - Achilles tendon
- sural nerve
- saphenous vein
- tail of epididymis
- epithelium of colon
- popliteal artery
- tibial arteries
- urethra
- right coronary artery
- vena cava
|
| | Top expressed in | - ascending aorta
- aortic valve
- conjunctival fornix
- zygote
- secondary oocyte
- iris
- otolith organ
- primary oocyte
- utricle
- hand
|
| | More reference expression data |
|
|---|
| BioGPS | 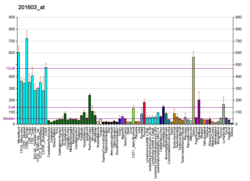

 | | More reference expression data |
|
|---|
|
| Gene ontology |
|---|
| Molecular function | - enzyme inhibitor activity
- 14-3-3 protein binding
- signal transducer activity
- protein binding
- protein kinase binding
- phosphatase regulator activity
| | Cellular component | - centrosome
- focal adhesion
- nucleoplasm
- Z disc
- PTW/PP1 phosphatase complex
- actin cytoskeleton
- contractile fiber
- A band
- kinetochore
- cytoplasm
- cytosol
- cytoskeleton
| | Biological process | - regulation of nucleocytoplasmic transport
- positive regulation of myosin-light-chain-phosphatase activity
- negative regulation of catalytic activity
- regulation of cell adhesion
- G2/M transition of mitotic cell cycle
- regulation of myosin-light-chain-phosphatase activity
- regulation of establishment of endothelial barrier
- positive regulation of transcription by RNA polymerase II
- signal transduction
- protein dephosphorylation
- mitotic cell cycle
- centrosome cycle
| | Sources:Amigo / QuickGO |
|
| Orthologs |
|---|
| Species | Human | Mouse |
|---|
| Entrez | | |
|---|
| Ensembl | | |
|---|
| UniProt | | |
|---|
| RefSeq (mRNA) | |
|---|
NM_001143885
NM_001143886
NM_001244990
NM_001244992
NM_002480 |
| |
|---|
NM_027892
NM_001368736
NM_001368737 |
|
|---|
| RefSeq (protein) | |
|---|
NP_001137357
NP_001137358
NP_001231919
NP_001231921
NP_002471 |
| |
|---|
NP_082168
NP_001355665
NP_001355666 |
|
|---|
| Location (UCSC) | Chr 12: 79.77 – 79.94 Mb | Chr 10: 108 – 108.12 Mb |
|---|
| PubMed search | [3] | [4] |
|---|
|
| Wikidata |
| View/Edit Human | View/Edit Mouse |
|
Protein phosphatase 1 regulatory subunit 12A is an enzyme that in humans is encoded by the PPP1R12A gene.[5][6]
Myosin phosphatase target subunit 1, which is also called the myosin-binding subunit of myosin phosphatase, is one of the subunits of myosin phosphatase. Myosin phosphatase regulates the interaction of actin and myosin downstream of the guanosine triphosphatase Rho. The small guanosine triphosphatase Rho is implicated in myosin light chain (MLC) phosphorylation, which results in contraction of smooth muscle [7] and interaction of actin and myosin in nonmuscle cells. The guanosine triphosphate (GTP)-bound, active form of RhoA (GTP.RhoA) specifically interacted with the myosin-binding subunit (MBS) of myosin phosphatase, which regulates the extent of phosphorylation of MLC. Rho-associated kinase (Rho-kinase), which is activated by GTP. RhoA, phosphorylated MBS and consequently inactivated myosin phosphatase. Overexpression of RhoA or activated RhoA in NIH 3T3 cells increased phosphorylation of MBS and MLC. Thus, Rho appears to inhibit myosin phosphatase through the action of Rho-kinase.[6]
Interactions
PPP1R12A has been shown to interact with Interleukin 16.[8]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000058272 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000019907 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Takahashi N, Ito M, Tanaka J, Nakano T, Kaibuchi K, Odai H, Takemura K (Nov 1997). "Localization of the gene coding for myosin phosphatase, target subunit 1 (MYPT1) to human chromosome 12q15-q21". Genomics. 44 (1): 150–2. doi:10.1006/geno.1997.4859. PMID 9286714.
- ^ a b "Entrez Gene: PPP1R12A protein phosphatase 1, regulatory (inhibitor) subunit 12A".
- ^ Michael P. Walsh; et al. "Thromboxane A2-induced contraction of rat caudal arterial smooth muscle involves activation of Ca2+ entry and Ca2+sensitization: Rho-associated kinase-mediated phosphorylation of MYPT1 at Thr-855 but not Thr-697" (PDF). Archived from the original (PDF) on 2011-07-13.
- ^ Bannert, Norbert; Vollhardt Karin; Asomuddinov Bakhtier; Haag Marion; König Herbert; Norley Stephen; Kurth Reinhard (Oct 2003). "PDZ Domain-mediated interaction of interleukin-16 precursor proteins with myosin phosphatase targeting subunits". J. Biol. Chem. 278 (43). United States: 42190–9. doi:10.1074/jbc.M306669200. ISSN 0021-9258. PMID 12923170.
Further reading
- Somlyo AP, Wu X, Walker LA, Somlyo AV (1999). "Pharmacomechanical coupling: the role of calcium, G-proteins, kinases and phosphatases". Rev. Physiol. Biochem. Pharmacol. 134: 201–34. doi:10.1007/3-540-64753-8_5. ISBN 978-3-540-64753-9. PMID 10087910.
- Ziter FA, Wiser WC, Robinson A (1977). "Three-generation pedigree of a Möbius syndrome variant with chromosome translocation". Arch. Neurol. 34 (7): 437–42. doi:10.1001/archneur.1977.00500190071011. PMID 880069.
- Slee JJ, Smart RD, Viljoen DL (1991). "Deletion of chromosome 13 in Moebius syndrome". J. Med. Genet. 28 (6): 413–4. doi:10.1136/jmg.28.6.413. PMC 1016909. PMID 1870098.
- Kimura K, Ito M, Amano M, et al. (1996). "Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase)". Science. 273 (5272): 245–8. Bibcode:1996Sci...273..245K. doi:10.1126/science.273.5272.245. PMID 8662509. S2CID 37249779.
- Ito M, Feng J, Tsujino S, et al. (1997). "Interaction of smooth muscle myosin phosphatase with phospholipids". Biochemistry. 36 (24): 7607–14. doi:10.1021/bi9702647. PMID 9200713.
- Nakai K, Suzuki Y, Kihira H, et al. (1997). "Regulation of myosin phosphatase through phosphorylation of the myosin-binding subunit in platelet activation". Blood. 90 (10): 3936–42. doi:10.1182/blood.V90.10.3936. PMID 9354661.
- Somlyo AP (1999). "Kinases, myosin phosphatase and Rho proteins: curiouser and curiouser". J. Physiol. 516 (3): 630. doi:10.1111/j.1469-7793.1999.0630u.x. PMC 2269296. PMID 10200412.
- Surks HK, Mochizuki N, Kasai Y, et al. (1999). "Regulation of myosin phosphatase by a specific interaction with cGMP- dependent protein kinase Ialpha". Science. 286 (5444): 1583–7. doi:10.1126/science.286.5444.1583. PMID 10567269.
- Feng J, Ito M, Ichikawa K, et al. (2000). "Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase". J. Biol. Chem. 274 (52): 37385–90. doi:10.1074/jbc.274.52.37385. PMID 10601309.
- Arimura T, Suematsu N, Zhou YB, et al. (2001). "Identification, characterization, and functional analysis of heart-specific myosin light chain phosphatase small subunit". J. Biol. Chem. 276 (9): 6073–82. doi:10.1074/jbc.M008566200. PMID 11067852.
- Sebbagh M, Renvoizé C, Hamelin J, et al. (2001). "Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing". Nat. Cell Biol. 3 (4): 346–52. doi:10.1038/35070019. PMID 11283607. S2CID 36187702.
- Murányi A, Zhang R, Liu F, et al. (2001). "Myotonic dystrophy protein kinase phosphorylates the myosin phosphatase targeting subunit and inhibits myosin phosphatase activity". FEBS Lett. 493 (2–3): 80–4. doi:10.1016/S0014-5793(01)02283-9. PMID 11287000. S2CID 26428853.
- Machida H, Ito M, Okamoto R, et al. (2001). "Molecular cloning and analysis of the 5'-flanking region of the human MYPT1 gene". Biochim. Biophys. Acta. 1517 (3): 424–9. doi:10.1016/s0167-4781(00)00285-2. PMID 11342221.
- Kiss E, Murányi A, Csortos C, et al. (2002). "Integrin-linked kinase phosphorylates the myosin phosphatase target subunit at the inhibitory site in platelet cytoskeleton". Biochem. J. 365 (Pt 1): 79–87. doi:10.1042/BJ20011295. PMC 1222641. PMID 11931630.
- Velasco G, Armstrong C, Morrice N, et al. (2002). "Phosphorylation of the regulatory subunit of smooth muscle protein phosphatase 1M at Thr850 induces its dissociation from myosin". FEBS Lett. 527 (1–3): 101–4. doi:10.1016/S0014-5793(02)03175-7. PMID 12220642. S2CID 39366441.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. Bibcode:2002PNAS...9916899M. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Kitazawa T, Eto M, Woodsome TP, Khalequzzaman M (2003). "Phosphorylation of the myosin phosphatase targeting subunit and CPI-17 during Ca2+ sensitization in rabbit smooth muscle". J. Physiol. 546 (Pt 3): 879–89. doi:10.1113/jphysiol.2002.029306. PMC 2342583. PMID 12563012.
- Seko T, Ito M, Kureishi Y, et al. (2003). "Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle". Circ. Res. 92 (4): 411–8. doi:10.1161/01.RES.0000059987.90200.44. PMID 12600888.
External links
- PPP1R12A Info with links in the Cell Migration Gateway Archived 2014-12-11 at the Wayback Machine
 | This article on a gene on human chromosome 12 is a stub. You can help Wikipedia by expanding it. |

 1s70: Complex between protein ser/thr phosphatase-1 (delta) and the myosin phosphatase targeting subunit 1 (MYPT1)
1s70: Complex between protein ser/thr phosphatase-1 (delta) and the myosin phosphatase targeting subunit 1 (MYPT1)




















