Glutathione S-transferase A1
| GSTA1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | GSTA1, GST2, GSTA1-1, GTH1, GST-epsilon, glutathione S-transferase alpha 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 138359; MGI: 95863; HomoloGene: 130684; GeneCards: GSTA1; OMA:GSTA1 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Glutathione S-transferase A1 is an enzyme that in humans is encoded by the GSTA1 gene.[5]
Cytosolic and membrane-bound forms of glutathione S-transferase are encoded by two distinct supergene families. These enzymes function in the detoxification of electrophilic compounds, including carcinogens, therapeutic drugs, environmental toxins and products of oxidative stress, by conjugation with glutathione. The genes encoding these enzymes are known to be highly polymorphic. These genetic variations can change an individual's susceptibility to carcinogens and toxins as well as affect the toxicity and efficacy of some drugs. At present, eight distinct classes of the soluble cytoplasmic mammalian glutathione S-transferases have been identified: alpha, kappa, mu, omega, pi, sigma, theta and zeta. This gene encodes a glutathione S-transferase belonging to the alpha class. The alpha class genes, located in a cluster mapped to chromosome 6, are the most abundantly expressed glutathione S-transferases in liver (hepatocytes) and kidney (proximal tubules). In addition to metabolizing bilirubin and certain anti-cancer drugs in the liver, the alpha class of these enzymes exhibit glutathione peroxidase activity, thereby protecting the cells from reactive oxygen species and the products of peroxidation.[6]
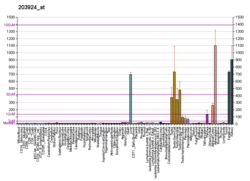
Release of GST-A1 as an indication of cellular necrosis
Increases in serum and urinary GST-A1 have been found in association with hepatocyte and renal proximal tubular necrosis respectively, and have potential for monitoring injury to these tissues.[7][8]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000243955 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000057933 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Mucher G, Becker J, Knapp M, Buttner R, Moser M, Rudnik-Schoneborn S, Somlo S, Germino G, Onuchic L, Avner E, Guay-Woodford L, Zerres K (Apr 1998). "Fine mapping of the autosomal recessive polycystic kidney disease locus (PKHD1) and the genes MUT, RDS, CSNK2 beta, and GSTA1 at 6p21.1-p12". Genomics. 48 (1): 40–5. doi:10.1006/geno.1997.5145. PMID 9503014.
- ^ "Entrez Gene: GSTA1 glutathione S-transferase A1".
- ^ Knapen, MF; Mulder, TP; Bisseling, JG; Penders, RH; Peters, WH; Steegers, EA (January 1998). "Plasma glutathione S-transferase alpha 1-1: a more sensitive marker for hepatocellular damage than serum alanine aminotransferase in hypertensive disorders of pregnancy". American Journal of Obstetrics and Gynecology. 178 (1 Pt 1): 161–5. doi:10.1016/S0002-9378(98)70645-3. PMID 9465822.
- ^ Heemskerk, S; Pickkers, P; Bouw, MP; Draisma, A; van der Hoeven, JG; Peters, WH; Smits, P; Russel, FG; Masereeuw, R (July 2006). "Upregulation of renal inducible nitric oxide synthase during human endotoxemia and sepsis is associated with proximal tubule injury". Clinical Journal of the American Society of Nephrology. 1 (4): 853–62. doi:10.2215/cjn.00490206. PMID 17699297.
Further reading
- Morel F, Schulz WA, Sies H (1995). "Gene structure and regulation of expression of human glutathione S-transferases alpha". Biol. Chem. Hoppe-Seyler. 375 (10): 641–9. PMID 7888077.
- Stenberg G, Björnestedt R, Mannervik B (1992). "Heterologous expression of recombinant human glutathione transferase A1-1 from a hepatoma cell line". Protein Expr. Purif. 3 (1): 80–4. doi:10.1016/1046-5928(92)90060-A. PMID 1330133.
- Bogaards JJ, van Ommen B, van Bladeren PJ (1992). "Purification and characterization of eight glutathione S-transferase isoenzymes of hamster. Comparison of subunit composition of enzymes from liver, kidney, testis, pancreas and trachea". Biochem. J. 286 (2): 383–8. doi:10.1042/bj2860383. PMC 1132909. PMID 1530570.
- Rozen F, Nguyen T, Pickett CB (1992). "Isolation and characterization of a human glutathione S-transferase Ha1 subunit gene". Arch. Biochem. Biophys. 292 (2): 589–93. doi:10.1016/0003-9861(92)90035-U. PMID 1731620.
- Board PG, Mannervik B (1991). "The contribution of the C-terminal sequence to the catalytic activity of GST2, a human alpha-class glutathione transferase". Biochem. J. 275 (1): 171–4. doi:10.1042/bj2750171. PMC 1150028. PMID 2018473.
- Hayes JD, Kerr LA, Cronshaw AD (1990). "Evidence that glutathione S-transferases B1B1 and B2B2 are the products of separate genes and that their expression in human liver is subject to inter-individual variation. Molecular relationships between the B1 and B2 subunits and other Alpha class glutathione S-transferases". Biochem. J. 264 (2): 437–45. doi:10.1042/bj2640437. PMC 1133600. PMID 2604726.
- Board PG, Webb GC (1987). "Isolation of a cDNA clone and localization of human glutathione S-transferase 2 genes to chromosome band 6p12". Proc. Natl. Acad. Sci. U.S.A. 84 (8): 2377–81. Bibcode:1987PNAS...84.2377B. doi:10.1073/pnas.84.8.2377. PMC 304654. PMID 3031680.
- Rhoads DM, Zarlengo RP, Tu CP (1987). "The basic glutathione S-transferases from human livers are products of separate genes". Biochem. Biophys. Res. Commun. 145 (1): 474–81. doi:10.1016/0006-291X(87)91345-3. PMID 3036131.
- Chow NW, Whang-Peng J, Kao-Shan CS, et al. (1988). "Human glutathione S-transferases. The Ha multigene family encodes products of different but overlapping substrate specificities". J. Biol. Chem. 263 (26): 12797–800. doi:10.1016/S0021-9258(18)37626-9. PMID 3138230.
- Tu CP, Qian B (1988). "Nucleotide sequence of the human liver glutathione S-transferase subunit 1 cDNA". Biochem. Soc. Trans. 15 (4): 734–6. doi:10.1042/bst0150734. PMID 3678589.
- Tu CP, Qian B (1987). "Human liver glutathione S-transferases: complete primary sequence of an Ha subunit cDNA". Biochem. Biophys. Res. Commun. 141 (1): 229–37. doi:10.1016/S0006-291X(86)80358-8. PMID 3800996.
- Suzuki T, Smith S, Board PG (1994). "Structure and function of the 5' flanking sequences of the human alpha class glutathione S-transferase genes". Biochem. Biophys. Res. Commun. 200 (3): 1665–71. doi:10.1006/bbrc.1994.1643. PMID 8185623.
- Anttila S, Hirvonen A, Vainio H, et al. (1994). "Immunohistochemical localization of glutathione S-transferases in human lung". Cancer Res. 53 (23): 5643–8. PMID 8242618.
- Suzuki T, Johnston PN, Board PG (1994). "Structure and organization of the human alpha class glutathione S-transferase genes and related pseudogenes". Genomics. 18 (3): 680–6. doi:10.1016/S0888-7543(05)80373-8. PMID 8307579.
- Sinning I, Kleywegt GJ, Cowan SW, et al. (1993). "Structure determination and refinement of human alpha class glutathione transferase A1-1, and a comparison with the Mu and Pi class enzymes". J. Mol. Biol. 232 (1): 192–212. doi:10.1006/jmbi.1993.1376. PMID 8331657.
- Ahmad H, Singhal SS, Saxena M, Awasthi YC (1993). "Characterization of two novel subunits of the alpha-class glutathione S-transferases of human liver". Biochim. Biophys. Acta. 1161 (2–3): 333–6. doi:10.1016/0167-4838(93)90234-i. PMID 8431482.
- Cameron AD, Sinning I, L'Hermite G, et al. (1996). "Structural analysis of human alpha-class glutathione transferase A1-1 in the apo-form and in complexes with ethacrynic acid and its glutathione conjugate". Structure. 3 (7): 717–27. doi:10.1016/S0969-2126(01)00206-4. PMID 8591048.
- Mulder TP, Peters WH, Court DA, Jansen JB (1996). "Sandwich ELISA for glutathione S-transferase Alpha 1-1: plasma concentrations in controls and in patients with gastrointestinal disorders". Clin. Chem. 42 (3): 416–9. doi:10.1093/clinchem/42.3.416. PMID 8598105.
- v
- t
- e
-
 1ags: A SURFACE MUTANT (G82R) OF A HUMAN ALPHA-GLUTATHIONE S-TRANSFERASE SHOWS DECREASED THERMAL STABILITY AND A NEW MODE OF MOLECULAR ASSOCIATION IN THE CRYSTAL
1ags: A SURFACE MUTANT (G82R) OF A HUMAN ALPHA-GLUTATHIONE S-TRANSFERASE SHOWS DECREASED THERMAL STABILITY AND A NEW MODE OF MOLECULAR ASSOCIATION IN THE CRYSTAL -
 1gsd: GLUTATHIONE TRANSFERASE A1-1 IN UNLIGANDED FORM
1gsd: GLUTATHIONE TRANSFERASE A1-1 IN UNLIGANDED FORM -
 1gse: GLUTATHIONE TRANSFERASE A1-1 COMPLEXED WITH AN ETHACRYNIC ACID GLUTATHIONE CONJUGATE (MUTANT R15K)
1gse: GLUTATHIONE TRANSFERASE A1-1 COMPLEXED WITH AN ETHACRYNIC ACID GLUTATHIONE CONJUGATE (MUTANT R15K) -
 1gsf: GLUTATHIONE TRANSFERASE A1-1 COMPLEXED WITH ETHACRYNIC ACID
1gsf: GLUTATHIONE TRANSFERASE A1-1 COMPLEXED WITH ETHACRYNIC ACID -
 1guh: STRUCTURE DETERMINATION AND REFINEMENT OF HUMAN ALPHA CLASS GLUTATHIONE TRANSFERASE A1-1, AND A COMPARISON WITH THE MU AND PI CLASS ENZYMES
1guh: STRUCTURE DETERMINATION AND REFINEMENT OF HUMAN ALPHA CLASS GLUTATHIONE TRANSFERASE A1-1, AND A COMPARISON WITH THE MU AND PI CLASS ENZYMES -
 1k3l: Crystal Structure Analysis of S-hexyl-glutathione Complex of Glutathione Transferase at 1.5 Angstroms Resolution
1k3l: Crystal Structure Analysis of S-hexyl-glutathione Complex of Glutathione Transferase at 1.5 Angstroms Resolution -
 1k3o: Crystal Structure Analysis of apo Glutathione S-Transferase
1k3o: Crystal Structure Analysis of apo Glutathione S-Transferase -
 1k3y: Crystal Structure Analysis of human Glutathione S-transferase with S-hexyl glutatione and glycerol at 1.3 Angstrom
1k3y: Crystal Structure Analysis of human Glutathione S-transferase with S-hexyl glutatione and glycerol at 1.3 Angstrom -
 1pkw: Crystal structure of human glutathione transferase (GST) A1-1 in complex with glutathione
1pkw: Crystal structure of human glutathione transferase (GST) A1-1 in complex with glutathione -
 1pkz: Crystal structure of human glutathione transferase (GST) A1-1
1pkz: Crystal structure of human glutathione transferase (GST) A1-1 -
 1pl1: Crystal structure of human glutathione transferase (GST) A1-1 in complex with a decarboxy-glutathione
1pl1: Crystal structure of human glutathione transferase (GST) A1-1 in complex with a decarboxy-glutathione -
 1pl2: Crystal structure of human glutathione transferase (GST) A1-1 T68E mutant in complex with decarboxy-glutathione
1pl2: Crystal structure of human glutathione transferase (GST) A1-1 T68E mutant in complex with decarboxy-glutathione -
 1tdi: Crystal Structure of hGSTA3-3 in Complex with Glutathione
1tdi: Crystal Structure of hGSTA3-3 in Complex with Glutathione -
 1usb: RATIONAL DESIGN OF A NOVEL ENZYME - EFFICIENT THIOESTER HYDROLYSIS ENABLED BY THE INCORPORATION OF A SINGLE HIS RESIDUE INTO HUMAN GLUTATHIONE TRANSFERASE A1-1
1usb: RATIONAL DESIGN OF A NOVEL ENZYME - EFFICIENT THIOESTER HYDROLYSIS ENABLED BY THE INCORPORATION OF A SINGLE HIS RESIDUE INTO HUMAN GLUTATHIONE TRANSFERASE A1-1 -
 1xwg: Human GST A1-1 T68E mutant
1xwg: Human GST A1-1 T68E mutant -
 1ydk: Crystal structure of the I219A mutant of human glutathione transferase A1-1 with S-hexylglutathione
1ydk: Crystal structure of the I219A mutant of human glutathione transferase A1-1 with S-hexylglutathione
 | This article on a gene on human chromosome 6 is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e