DFDT
 | |
| Names | |
|---|---|
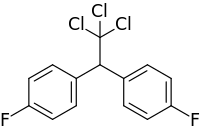
| Preferred IUPAC name 1,1′-(2,2,2-Trichloroethane-1,1-diyl)bis(4-fluorobenzene) | |
| Other names Fluorogesarol; Fluoro-DDT; p,p′-Fluoro-DDT; p,p′-Difluorodiphenyltrichloroethane | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.006.814 |
| EC Number |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C14H9Cl3F2 |
| Molar mass | 321.57 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Difluorodiphenyltrichloroethane (DFDT) is a chemical compound. Its composition is the same as that of the insecticide DDT, except that two of DDT's chlorine atoms are replaced by two fluorine atoms.[1]
DFDT was developed as an insecticide by German scientists during World War II, partly to avoid having to pay license fees for DDT. It was documented by Allied military intelligence, but remained in obscurity after the war.[1]
In 2019, New York University chemists reported that DFDT and a mono-fluorinated derivative, MFDT, might be a more effective insecticide than DDT, and might therefore be used to combat malaria with less of an environmental impact.[1][2] A later study, however, found that "DFDT is unlikely to be a viable public health vector control insecticide".[3]
References
- ^ a b c Chang, Kenneth (17 October 2019). "A Nazi Version of DDT Was Forgotten. Could It Help Fight Malaria?". The New York Times. Retrieved 18 October 2019.
- ^ Zhu, Xiaolong; Hu, Chunhua T.; Yang, Jingxiang; Joyce, Leo A.; Qiu, Mengdi; Ward, Michael D.; Kahr, Bart (11 October 2019). "Manipulating Solid Forms of Contact Insecticides for Infectious Disease Prevention". Journal of the American Chemical Society. 141 (42): 16858–16864. doi:10.1021/jacs.9b08125. PMID 31601104. S2CID 204244148.
- ^ Norris, Edmund J.; Demares, Fabien; Zhu, Xiaolong; Bloomquist, Jeffrey R. (2020-11-01). "Mosquitocidal activity of p,p'-difluoro-diphenyl-trichloroethane (DFDT)". Pesticide Biochemistry and Physiology. 170: 104686. doi:10.1016/j.pestbp.2020.104686. ISSN 0048-3575. PMID 32980070. S2CID 222169601.
- v
- t
- e
- Aluminium phosphide
- Boric acid
- Chromated copper arsenate
- Copper(II) arsenate
- Copper(I) cyanide
- Cryolite
- Diatomaceous earth
- Lead hydrogen arsenate
- Paris Green
- Scheele's Green
- Acephate
- Azamethiphos
- Azinphos-methyl
- Bensulide
- Chlorethoxyfos
- Chlorfenvinphos
- Chlorpyrifos
- Chlorpyrifos-methyl
- Coumaphos
- Demeton-S-methyl
- Diazinon
- Dichlorvos
- Dicrotophos
- Diisopropyl fluorophosphate
- Dimefox
- Dimethoate
- Dioxathion
- Disulfoton
- Ethion
- Ethoprop
- Fenamiphos
- Fenitrothion
- Fenthion
- Fosthiazate
- Isoxathion
- Malathion
- Methamidophos
- Methidathion
- Mevinphos
- Mipafox
- Monocrotophos
- Naled
- Omethoate
- Oxydemeton-methyl
- Parathion
- Parathion-methyl
- Phenthoate
- Phorate
- Phosalone
- Phosmet
- Phoxim
- Pirimiphos-methyl
- Quinalphos
- R-16661
- Schradan
- Temefos
- Tebupirimfos
- Terbufos
- Tetrachlorvinphos
- Tribufos
- Trichlorfon
- Acrinathrin
- Allethrins
- Bifenthrin
- Bioallethrin
- Cyfluthrin
- Cyhalothrin
- Cypermethrin
- Cyphenothrin
- Deltamethrin
- Empenthrin
- Esfenvalerate
- Etofenprox
- Fenpropathrin
- Fenvalerate
- Flumethrin
- Fluvalinate
- Imiprothrin
- Metofluthrin
- Permethrin
- Phenothrin
- Prallethrin
- Pyrethrin (I, II; chrysanthemic acid)
- Pyrethrum
- Resmethrin
- Silafluofen
- Tefluthrin
- Tetramethrin
- Tralomethrin
- Transfluthrin
- Afoxolaner
- Amitraz
- Azadirachtin
- Bensultap
- Buprofezin
- Cartap
- Chlordimeform
- Chlorfenapyr
- Cyromazine
- Fenazaquin
- Fenoxycarb
- Fipronil
- Fluralaner
- Hydramethylnon
- Indoxacarb
- Limonene
- Lotilaner (+milbemycin oxime)
- Pyridaben
- Pyriprole
- Sarolaner
- Adjuvants (Piperonyl butoxide, Sesamex)
- Spinosad
- Sulfluramid
- Tebufenozide
- Tebufenpyrad
- Veracevine
- Xanthone
- Metaflumizone
 | This article about an organic halide is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e











